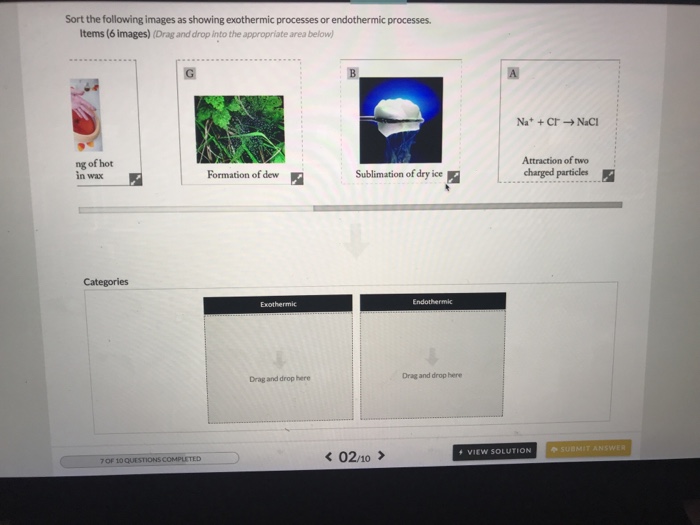
Is the Attraction of Two Charged Particles Endothermic or Exothermic
See the answer See the answer done loading. The law of conservation of energy states that in any physical or chemical process energy is neither created nor destroyed.

Chapter 9 Chemical Bonding I Lewis Theory Why Do Atoms Bond Processes Are Spontaneous If They Result In A System With Lower Potential Energy Chemical Ppt Download
Whether the overall process is exo- or endothermic depends on whether the bond-breaking energy is greater than the hydrogen-bond-forming energy or not.
. This forms the hydrogen bond. Breaking solvent-solvent attractions Endothermic or exothermic. Also what does exothermic reaction mean.
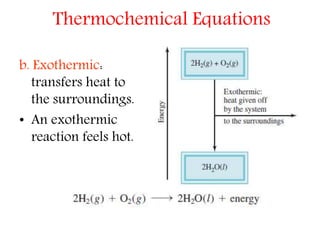
The formation of bonds is an exothermic process and. The opposite reaction in which heat energy is produced is an exothermic reaction. In endothermic reactions more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the products.
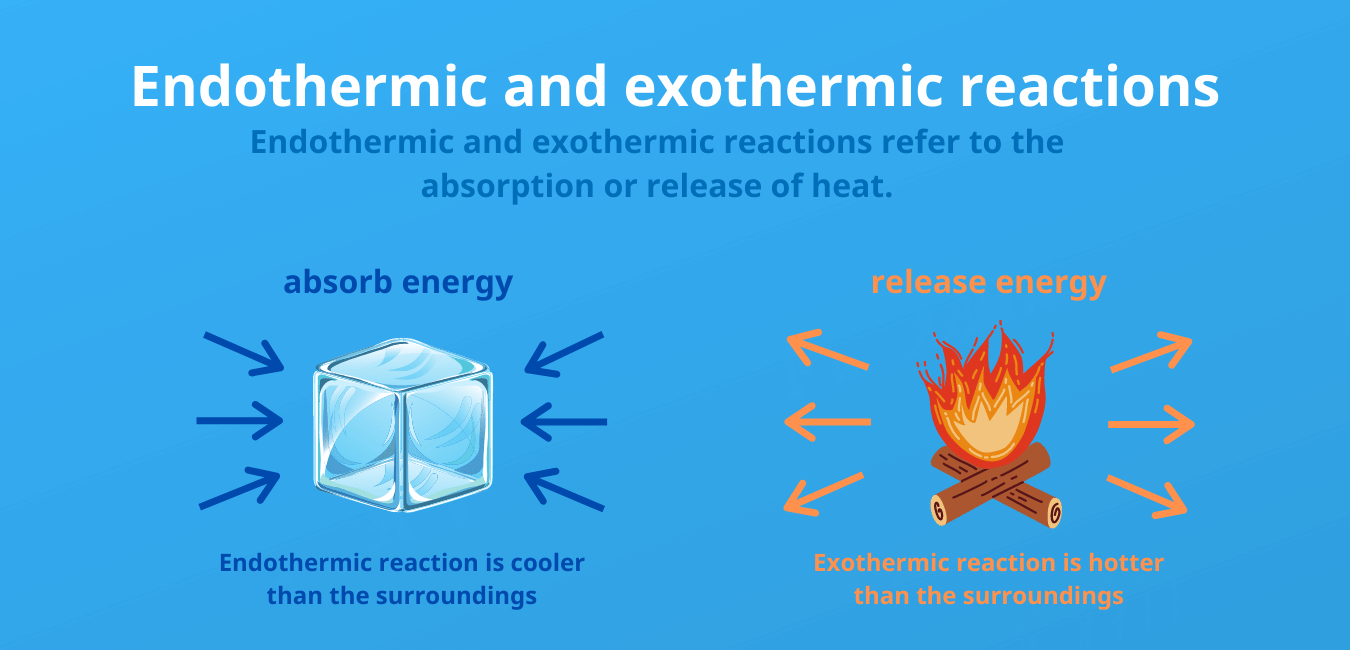
An endothermic reaction is a reaction in which heat energy is consumed. Exothermic and Endothermic Processes When physical or chemical changes occur they are generally accompanied by a transfer of energy. Which of the following processes are endothermic and which are exothermic.
The reverse reaction is not always endothermic or exothermic the reverse reaction is the opposite of whatever the initial reaction is so if. All forms of energy can be described as either exothermic or endothermic processes. An easy way to remember the difference between these two reaction types is by their prefixes.
When two oppositely charged particles are brought closer together under a Coulombic potential the potential energy of the pair decreases. Bond energy is the amount of energy absorbed to break the bonds or released energy during the formation of bonds in one mole of the substance. An exothermic reaction is a chemical reaction that releases energy through light or heat.
Endothermic processes as indicated by the prefix endo- are processes where heat is supplied to the system by the surroundings. The breaking of bonds is an endothermic process and needs to absorb an amount of energy from the surrounding So its ΔH has a positive sign. Endothermic vs Exothermic.
The exothermic reaction is the opposite of an endothermic reaction. Because the water molecules will solvate the charged atom and the molecule very easily--like magnesium sulfate. Hardeneing of hot paraffin wax.
It is the opposite of an endothermic reaction. Special water cases are seldom found. Endothermic reactions take in energy and the temperature of the surroundings decreases.
A few examples are neutralization burning a substance reactions of fuels deposition of dry ice respiration solution of sulfuric acid into water and much more. Solvation can be calculated using hydration energy. Endothermic processes as indicated by the prefix endo- are processes where heat is supplied to the system by the surroundings.
ΔH is just enthalpy or heat energy. When water dissolves a substance the water molecules attract and bond to the particles molecules or ions of the substance causing the particles to separate from each other. It releases energy by light or heat to its surrounding.
Endo- means to draw in and exo- means to give off. The positive Q reactions are. However when comparing two nonpolar gases the one with the greater London forces will generally be the more hydrophilic.
This causes an attraction to the two salt ions depending on their charge. A salt is a compound made up of positively charged ions and negatively charged ions which are held together in a solid state because the positive and negative charges attract one another. When an atom gains an electron there must be a net force of attraction between the atom and electron.
Chemical reactions that absorb or use energy overall are called endothermic. Endothermic Reaction For reactions in which there is an increase in the kinetic energy of the products Q is positive. Attraction of two charged particles.
One type of chemical process that can be either exothermic or endothermic is dissolving of salts in water. The energy of attraction is a type of energy since it is based on the position of the charged particles relative to each other. Attraction between opposite charges is exothermic.
Countless reactions are endothermic. A salt is a compound which is made up of positively charged and negatively charged ions which are held together in a solid state because the positive and negative charges attract each other. Melting of ice cream.
On the other hand if the energy released in step 2 is less than the energy absorbed in step 1 the dissolution process will be endothermic. The salt we put on our food is referred to as table salt and is a. If the energy released in step 2 is greater than the energy absorbed in step 1 the dissolution process will be exothermic.
Reactants products energy. The process of dissolving can be endothermic temperature goes down or exothermic temperature goes up. In the event of two mole ions drinking from a single cup of water hydration energy is produced or hydration enthalpy.
I can understand that it is easier for them to gain electrons than the others because of small size and increase in nuclear charge and I guess it just goes to the first question again. Breaking the bond in an oxygen molecule. Expressed in a chemical equation.
Table of contents why energy is released during hydrationis hydration endothermic or exothermichow does hydration energy. Endothermic vs Exothermic All forms of energy can be described as either exothermic or endothermic processes. This corresponds with a ΔH value.
Exothermic reactions are accompanied by an increase in temperature of the reaction mixture. This is called hydration. In thermodynamics these two types of reactions are classified as exothermic or endothermic respectively.
This latter bond formation releases energy and is therefore exothermic. However conservation of energy requires that this potential energy be converted into some other form total energy is conserved. When a reaction proceeds it either releases energy to or absorbs energy from its surroundings.
Difference Between Endothermic and Exothermic Reactions. If the solution is cold the reaction is endothermic but if the solution is hot the reaction is exothermic. In many of them the input of energy is used to break intermolecular and chemical bonds allowing for the formation of new products.
If the two particles have opposite charges as in ionic compounds the value of the energy will be meaning that energy is released by bringing the particles together.

Chapter 5 Thermochemistry Ppt Download

Thermochemistry Energy Changes In Reactions Ppt Download

Thermochemistry Energy Changes In Reactions Ppt Download
Chemical Energetics Chemistry Quizizz
How Does The Energy Contained In Chemical Bonds Change During Endothermic And Exothermic Reactions Quora

Solved Sort The Following Images As Showing Exothermic Chegg Com

Chapter 9 Chemical Bonding I Lewis Theory Why Do Atoms Bond Processes Are Spontaneous If They Result In A System With Lower Potential Energy Chemical Ppt Download

No comments for "Is the Attraction of Two Charged Particles Endothermic or Exothermic"
Post a Comment